GB Innomech Ltd
GB Innomech Ltd – Bespoke Automation SpecialistsGB Innomech excels at developing and producing innovative electro mechanical prototypes, special purpose machinery manufacturing, and bespoke automated equipment, improving our customers productivity, profitability and complianceCambridge based, with over 20 years’ experience producing feasibility studies, prototypes, bespoke automation control systems and automated assembly machinery for the pharmaceutical, medical device and other technological industry sectors, GB Innomech offers a committed team with experience in specialist automation including robot handling, PLC control, pick and place and vision systems, with skills in mechatronics, production automation, specialised automation control systems engineering, innovative bespoke design, and validation.
-
Innomech signs as ABB Robotics partner for next generation automation
8 July 2019GB Innomech (Innomech) has joined ABB Robotics global partner network with plans to integrate ABB’s high-performance robots into a new generation of flexible automated manufacturing systems. Innomech helps automate critical manufacturing and quality control processes for its clients and works in multiple sectors including pharmaceuticals, medical devices, automotive and food.
Read the complete Article Here
-
Innomech secures ISO 14001:2004 in record time
8 July 2019GB Innomech (Innomech) – specialists in the design and development of automation systems – has had its long-term corporate commitment to eco-friendly principles further endorsed by securing ISO
14001:2004
certification. The company has been running its own environmental management system for many years and as a result completed the in-depth audit and review process in just six months.
The decision to become officially recognised was taken because a growing number of Innomech’s clients are increasingly involved in their own corporate social responsibility programmes. ISO 14001 accreditation makes it easier for them to work with the company.
-
Innomech manager Mark runs London marathon for GOSH
8 July 2019Mark Seaman, development team manager at GB Innomech (Innomech) is preparing for the sporting challenge of his life – pounding the streets of London in aid of Great Ormond Street Hospital (GOSH).
47 year old Mark, who used to compete regularly in county schools championships, is in training to run the 2011 Virgin London Marathon following a challenge from his eldest son. 16 year old Zac thought his dad would never be able to make a marathon – five months later and Mark has never looked back. He completed the 13 mile adidas Silverstone Half Marathon in March in just 1 hour and 55 minutes and hopes to cross the finishing line in London in under four and a half hours.
-
Innomech to lead automation workshop at B2B life science event
8 July 2019Tim Mead, commercial director at GB Innomech (Innomech), has been invited to lead a round table workshop on automation at Bench-to-Boardroom (B2B), one of the UK’s most popular networking exhibitions for the life science and healthcare industry.
B2B is an intensive one day event – organised by One Nucleus – that combines a supplier exhibition with workshops and keynote presentations from thought leaders to help stimulate ideas, discussion and networking. This year’s event takes place on Tuesday 8 March at Newmarket Racecourse and will be opened by Sir Greg Winter, deputy director of MRC Laboratory of Molecular Biology. Innomech’s workshop starts at 2pm and is open to all exhibition attendees.
-
Innomech celebrates 21st birthday
8 July 2019Ely-based GB Innomech (Innomech) – which specialises in the design and development of advanced automation systems to improve manufacturing efficiency and costs – has celebrated 21 years of business success this week with a dinner for staff and their partners at Queens’ College, Cambridge.
“Everyone at tonight’s celebration has played a key role in bringing Innomech to where it is today. Our engineering, operations, commercial and management teams have never been stronger; in 2009 we secured The Queen’s Award for Enterprise for international trade; and we are extremely well positioned to make 2011 a record year for the business,” said David Beale, technical director and business founder.
-
Press release: Innomech trades technology for art with college sponsorship
8 July 2019GB Innomech (Innomech), which specialises in the design and development of advanced automation systems, is strengthening its community links and encouraging local students to develop their creativity through a unique tie up with nearby Witchford Village College (WVC). Innomech is providing the college’s art and design department with the very latest professional digital camera for use in GCSE course work and other projects, while the students have created artworks, focusing on the environment, to lend to the company.
-
Editorial: Quality without the Pain
8 July 2019Peter Woods, programme manager at GB Innomech describes new automated approaches in pharmaceutical manufacturing.
Pharmaceutical and medical device manufacturers are constantly focused on guaranteeing product quality and patient safety. The highly regulated environments in which such products are manufactured forces suppliers of automation in this market to themselves meet demanding levels of quality assurance, often associated with a significant overhead of documentation.
-
Press release: Innomech develops powerful ‘track and trace’ technology for healthcare markets
8 July 2019GB Innomech (Innomech), which specialises in the development of advanced automation systems, is helping develop a powerful new low-cost approach to uniquely mark pharmaceutical and related healthcare products and therefore improve product traceability. The technique will allow faster identification and resolution of any manufacturing quality problems but will also prove invaluable as an anti-counterfeit measure because the specific coding and validation systems are almost impossible to copy.
Currently most components within diagnostic kits, medical devices and other healthcare products and equipment are ‘stamped’ with a lot code at the point of manufacture. However, these codes are of limited use for quality improvement unless products are produced in very small batches. As a result, regulatory bodies across the world are now putting manufacturers under increasing pressure to invest in much more sophisticated traceability systems, while manufacturers are looking for effective ways to prevent the growing problem of counterfeiting of pharmaceuticals and other healthcare products.
-
Press release: Innomech teams up with biotechnology company for end-of-line test systems
8 July 2019One of the world’s leading biotechnology groups has appointed GB Innomech (Innomech) to develop a sophisticated, automated end-of-line testing system for a new drug delivery pen. Innomech was first asked to advise on the feasibility of automating an existing quality control process for the multi-dose, dial-a-dose injection pen before being appointed to design and build two machines.
Innomech’s new easy-to-use system incorporates calibrated measuring devices to check and record over 20 parameters on four key areas of the pen while simulating use of the assembled, multi-part injection device. For example, the system measures the forces required to fire the trigger or adjust the dose control knob before comparing them against agreed tolerances to confirm products comply fully with the required specification. The fully automated test routine takes approximately 30 seconds to complete.
-
Editorial: Top tips for successful manufacturing development
8 July 2019One of the toughest challenges in bringing innovative products to market is bridging the gap between ‘proof of principle’ and volume manufacture. And it’s a massive hurdle for early-stage businesses who are often integrating new materials, functions and technologies for the first time.
Part or fully-automated production systems can deliver perfect products at the lowest possible price but machine development needs careful managing. Here are top ten tips to get you started:
-
Press release: Innomech celebrates 8 year partnership with Capsugel
8 July 2019GB Innomech (Innomech) is today celebrating the eighth anniversary of a successful commercial partnership with Capsugel® that started with a one-off requirement to build a precision powder micro-dosing system for the pharmaceutical industry.
Innomech was first appointed in 2001 to advise on and help develop a commercial machine based on the Xcelodose patented proprietary micro-dosing technology and system concepts. The first Xcelodose® Precision Powder Micro-dosing Systems proved so successful that the company was retained to build and install further units.
Capsugel’s patented Xcelodose system is now used routinely by most major pharmaceutical companies and contract research organisations to automatically fill capsules with pure pharmaceutical powders for use in clinical trials or for small-scale production. In addition to offering repeat build, Innomech now provides full installation, on-site training, testing and after-sales support for the Xcelodose system.
-
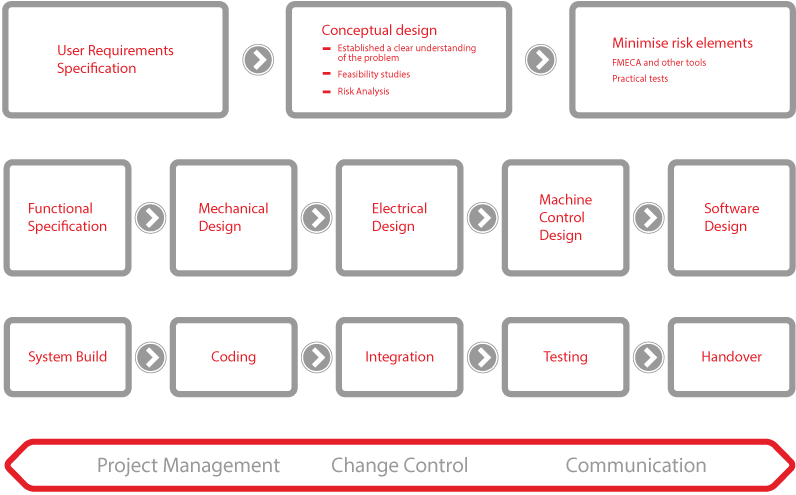
Editorial: The benefits of risk-based analyses during validated system development
8 July 2019In this article, we discuss the value of a properly focussed analysis of engineering risk in the design of automated manufacture and test systems for pharmaceutical applications, and illustrate our case with real examples seen in the manufacture of medical devices and in the design of machines used in drug development.
Introduction
In bringing new pharmaceutical products or medical devices to market, producers must adhere to rigorously documented procedures overseen by regulatory bodies, as embodied in for example ASTM E-2500 [1] and GAMP 5 [2], as well as maintaining a self-regulated quality system as in ISO 13485/ISO 9001.Typically, producers are dependent on third parties to supply instruments and systems for manufacture. The producer must demonstrate that their production process, including these third-party elements, meets necessary standards often involving formal validation. Consequently, producers expect that any third-party systems must themselves be demonstrated to be built to purpose, compliant to the same methodologies mentioned above.
-
Editorial: Working towards a cure for automation headaches
8 July 2019Introducing bespoke automation means a commitment to a high capital value investment and facing up to some degree of technical risk. Fortunately, techniques borrowed from medical device and pharmaceutical product validation can help ensure the outcome is positive for all concerned.
Few would argue that automation offers compelling benefits in productivity, consistency, and cost effectiveness. That being said, engineering a new process or automating a previously manual operation requires both careful management and an understanding of the potential pitfalls in converting a user’s aspiration into a piece of machinery.
-
Press release: Innomech secures The Queen’s Award for Enterprise
8 July 2019GB Innomech (Innomech) – a company specialising in the development of advanced automation systems for the pharmaceutical, medical device and environmental sectors – has been awarded The Queen’s Award for Enterprise 2009 in the international trade category on the back of soaring international sales.
76% of the company’s total sales in 2008 were overseas, an increase of 69% over 2006, at a time when official statistics show general UK manufacturing dropping at its fastest rate since records began over 40 years ago.
Innomech specialises in automating highly complex and labour-intensive manufacturing, inspection and testing processes in production plants to improve quality, increase throughput and reduce costs. The company works with multiple technologies but has particular expertise in precision powder handling and dispensing, widely used in the pharmaceutical industry as well as many other sectors.
-
Test station streamlines R&D programme
23 April 2018Automation consultancy Innomech has designed and developed an innovative new test station for Fluidic Analytics, the Cambridge-based protein analysis company, as a labour-saving R&D tool to help process multiple test samples and to ensure product quality.
The new test station works alongside the company's Fluidity systems for protein sizing and quantification and will be used for ongoing R&D studies targeting new protein characterisation applications and for product development. It will also be used to improve the efficiency of final performance testing of production units before shipping them to customers.
The new station automates a series of time-consuming and repetitive liquid handling and sample processing steps that are required when analysing multiple protein samples. The automated system, which is based around a Tecan Cavro Omni Flex liquid-handling robot, is programmed to sample a protein solution into a disposable chip before transferring it into a reader. The robot then waits for the analysis to complete before returning the chip to its original location.
"Innomech's new test station will be invaluable in helping us to expand and improve the operational efficiency of our in-house R&D, as well as streamlining final performance testing of our production units. The Innomech team has provided us with excellent design and engineering support for this automation project: they have worked closely to help define our requirements and to advise on the fastest and most cost-effective way of delivering them," says Andrew Lynn, CEO at Fluidic Analytics.
The automated test station has been designed to process up to 96 protein samples in a single run, without any operator involvement and to ensure sample handling errors are avoided throughout. The system can also run over 24 hours enabling samples to be processed overnight, which will help Fluidic Analytics to expand its R&D at minimal cost and to accelerate the development of new chip designs or instrument features for more advanced applications.
The new test station will also be used to process and analyse multiple standard samples to determine instrument performance and as one of a number of final performance checks on production units.
-
Innomech Delivers for Aston Particle Size
25 October 2017APT is developing a one-step particle engineering technology to enable ‘challenging’ active pharmaceutical ingredients to be dry powder coated onto carrier particles: without needing heat, solvents or pressure. Target applications for this processing technology breakthrough include the development of new dry powder inhaler formulations, improving the solubility of poorly water-soluble drugs, and developing taste-masking solutions for bitter-tasting drugs.
Innomech has designed the new easy-to-use system to process up to two kilograms of pharmaceutical powders which is a 100-fold increase on the capacity of APT’s existing lab-scale, proof of principle equipment. The system provides a fluidised environment for particle contact and adsorption of cohesive material over coarse particles without the need for solvent and heat. Pressure and temperature sensors, and an ultra-efficient heat exchanger are used to monitor and maintain the required process conditions. http://www.innomech.co.uk/category/blog